Have you ever wondered why ice cubes float in your glass of water? You might think that solid things, like ice, should sink, but ice behaves differently. This curious behaviour happens because of something called density. Let’s dive into the science behind it and learn why ice floats on water!

What is Density?
To understand why ice floats, we need to first understand density. Density is a measure of how much “stuff” (matter) is packed into a certain space (volume). Think of it like this: if you have two objects of the same size, but one is heavier than the other, the heavier one is denser. For example, a rock is denser than a sponge, which is why a rock sinks in water while a sponge might float.

Water and Its Special Properties
Water is a very special substance. It’s made up of tiny molecules, each consisting of two hydrogen atoms and one oxygen atom, giving it the chemical formula H₂O. These water molecules are constantly moving around, and how they move and arrange themselves depends on the temperature.
When water is a liquid, its molecules are closely packed but still have room to move around. As the water cools down and starts to freeze, its molecules slow down and begin to form a crystal-like structure called ice. Here’s where the magic happens: when water freezes, it expands!

The Science of Ice Floating
Now, why does this expansion cause ice to float? When water turns into ice, the molecules arrange themselves in a way that takes up more space. Even though it’s still made of the same molecules, they are spread out more, making ice less dense than liquid water.
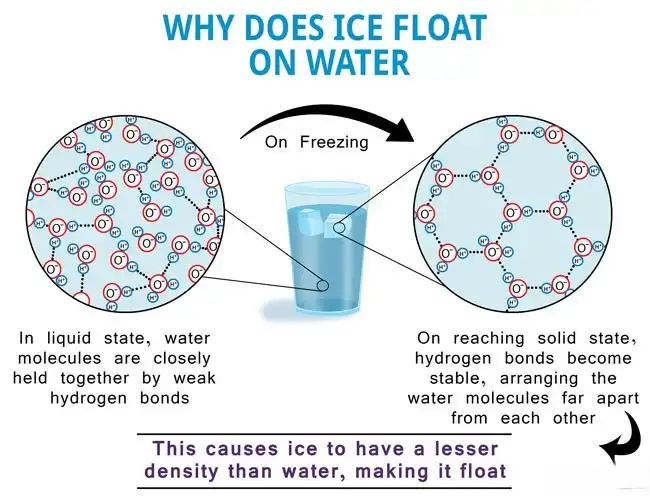
Let’s compare it to a real-life example: imagine you’re trying to fit a puzzle. If you tightly pack all the pieces together, it takes up less space. But if you leave gaps between the pieces, the puzzle would need more room. Similarly, when water turns into ice, it “leaves gaps” between the molecules, increasing its volume.

Because ice is less dense than water, it floats! In fact, about 9% of an ice cube stays above water while the rest remains submerged.

How Density and Buoyancy Work Together
The reason why ice floats has to do with buoyancy. This is a force that pushes objects up when they’re in a fluid, like water. For an object to float, the buoyant force pushing it up has to be stronger than the force pulling it down (its weight). Since ice is less dense than water, the buoyant force on ice is strong enough to keep it floating.

This is similar to how a boat floats. Even though a boat might be heavy, its shape allows it to displace enough water to create an upward buoyant force, allowing it to stay afloat.

Why Does Ice Expand When It Freezes?
Let’s take a closer look at the freezing process. Water freezes at 0°C (32°F). The water molecules start forming hydrogen bonds as they get colder and approach the freezing point. These are special connections between water molecules that lock them into place in a hexagonal shape.

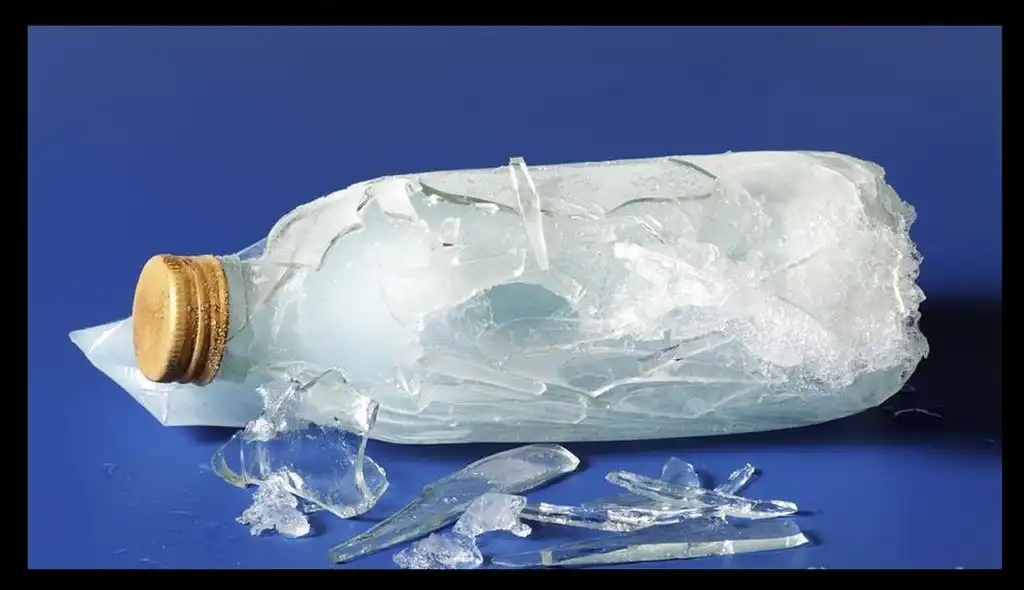
This hexagonal structure takes up more space than when the water molecules move freely in liquid form. Because of this, ice expands when it freezes. If you’ve ever left a bottle of water in the freezer for too long, you might have noticed that the water expands and sometimes even breaks the bottle!
What If Ice Didn’t Float?
Imagine a world where ice didn’t float. Ponds, lakes, and even oceans would freeze from the bottom up. During winter, the surface of lakes and ponds would be covered with ice, but if ice sank, the ice would accumulate at the bottom. Eventually, entire bodies of water could freeze solid!

Thankfully, because ice floats, only the top layer of a lake or pond freezes, while the water below stays warmer. This helps protect aquatic life during cold months, allowing fish and other creatures to survive in the water beneath the ice.

Why Does Ice in Your Drink Float?
When you drop ice into a glass of water or juice, it floats for the same reason – the ice is less dense than the liquid. As the ice begins to melt, the water from the ice mixes with the liquid in the glass. That’s why, after a while, the ice cubes get smaller and eventually disappear!

Floating Ice in Nature
This unique property of water has huge implications in nature. One of the most striking examples is seen in the Arctic and Antarctic regions, where massive icebergs float in the oceans. Like the ice cubes in your glass, these icebergs float because they are less dense than the salty ocean water. Interestingly, about 90% of an iceberg is submerged, meaning only 10% is visible above the water!

This is why ships must be cautious when navigating icy waters – the part of the iceberg hidden beneath the surface is much larger than what can be seen!

A Fun Experiment to Try
You can easily explore this concept at home! Here’s a simple experiment you can try:
- Fill a glass with water.
- Drop an ice cube into the glass.
- Watch how the ice cube floats with most of it underwater and just a small part above the surface.

Now for the fun part:
- Try adding salt to the water. Does the ice cube still float in the same way?
- Try the same experiment with other liquids, like juice or soda. Does the ice float differently?

You’ll notice that the ice cube may float higher in salt water because the salt increases the water’s density, making it easier for the ice to float!
Key Takeaways
- Ice floats on water because it is less dense than liquid water.
- When water freezes, it expands due to the formation of a hexagonal structure in the ice.
- The buoyant force pushes the ice up, keeping it afloat.
- This property of water is crucial for life in cold climates, as it prevents lakes and oceans from freezing solid.
Conclusion
The next time you sip a cold drink with ice, remember that those floating ice cubes are doing something amazing! They are showing us one of the most fascinating properties of water – the ability to expand and become less dense when frozen. This simple yet important behaviour helps life survive in cold environments and makes our planet a unique and wonderful place.
For more interesting articles, please visit www.kidzherald.com





