Have you ever wondered why wood doesn’t melt like ice or chocolate when you heat it? It’s an interesting question that takes us deep into the science of what wood is made of and how it reacts to heat. Let’s explore the reasons in a way that’s easy to understand, even if you’re not a scientist yet!

What Happens When You Heat Wood?
When you heat something like ice, it melts into water. Ice is made of water molecules held together in a solid form. When you heat it, the molecules gain energy and start moving around, turning it into a liquid.
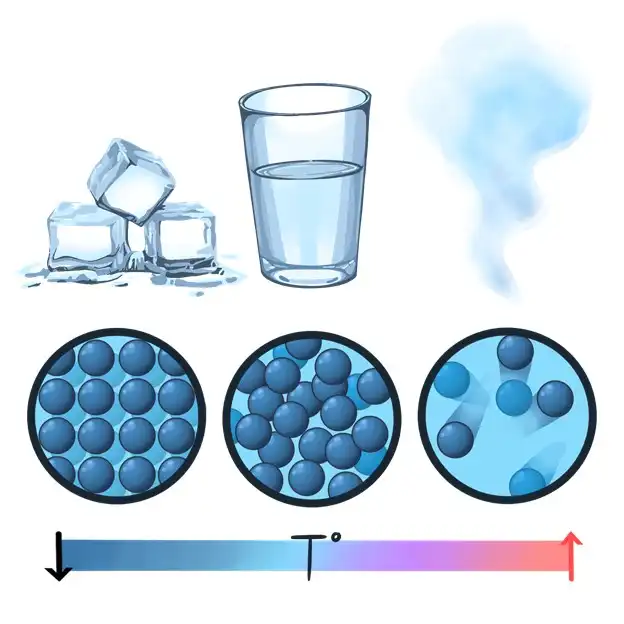
Wood, however, doesn’t behave the same way. When you heat wood, it doesn’t turn into a liquid. Instead, it burns, chars, or even turns into ash. This is because wood is made of complex substances that break apart when heated rather than melting.

What Is Wood Made Of?
Wood is mostly made up of three main substances:
- Cellulose: This gives wood its structure and helps it grow strong.
- Hemicellulose: Another part that supports the structure, but it’s more flexible.
- Lignin: This acts like glue, holding everything together.
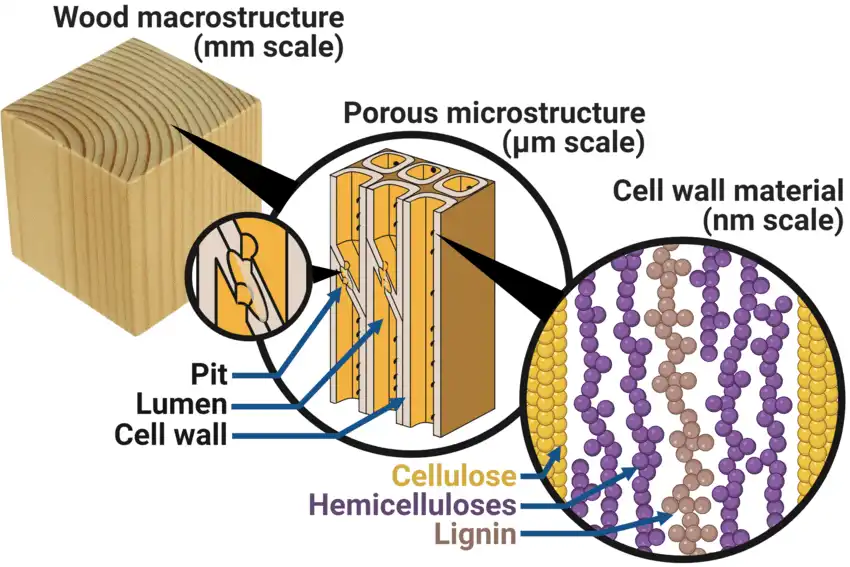
These substances are polymers, which are long chains of molecules. Unlike water, they don’t have a melting point. Instead, they decompose when they’re heated.
Why Doesn’t Wood Melt?
- Complex Structure: The polymers in wood are very complex. They don’t just soften or turn into liquid; they start to break apart when they get too hot.

- Decomposition: When wood is heated, it undergoes pyrolysis, which means the heat breaks the chemical bonds in it. Instead of melting, the wood breaks down into gases, liquids, and solid charcoal.

- No Freezing or Boiling Point: Unlike water, which freezes and boils at specific temperatures, the substances in wood don’t have a clear point where they change from solid to liquid. Instead, they chemically change into something else entirely.
What About Other Materials?
Many materials behave like wood when heated. For example:
- Paper: Made from cellulose, paper burns rather than melting.

- Plastic: Some plastics melt, but others decompose like wood because they are made of polymers too.

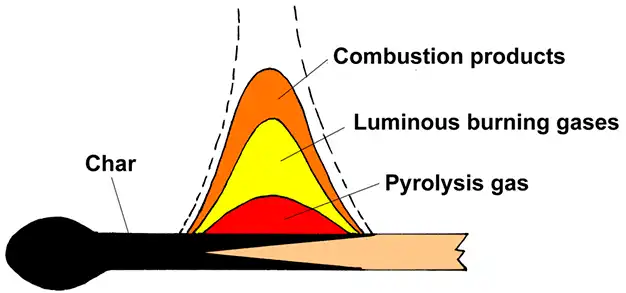
What Happens When Wood Burns?
When you heat wood enough, it burns. Here’s what happens step by step:

- Water Evaporates: Wood contains moisture. Heat first dries the wood by evaporating the water.
- Pyrolysis Begins: The heat breaks the chemical bonds in wood, releasing gases like carbon dioxide and methane.
- Flames Appear: The gases ignite, creating flames.
- Charcoal Forms: What’s left behind is solid charcoal, which can continue to burn until it turns into ash.
Can You Ever Melt Wood?
The short answer is no. Scientists have tried heating wood in special conditions, but it always decomposes before it can turn into a liquid. Even with advanced technology, melting wood remains impossible because of its unique chemical makeup.

Fun Facts About Wood
- Wood’s Strength: The cellulose in wood is so strong that it’s used to make paper, cardboard, and even some types of fabric!

- Eco-Friendly Material: Wood is renewable and biodegradable, making it one of the most environmentally friendly materials.

- Wood Gas: During pyrolysis, the gases released from wood can be used as fuel.
Why Is This Important?
Understanding why wood doesn’t melt teaches us a lot about the materials around us. It helps scientists and engineers design better materials and find new ways to use wood. Plus, it’s just cool to know why campfires work the way they do!

Conclusion:-Wood doesn’t melt because of its complex structure and chemical composition. Instead of turning into a liquid, it burns and decomposes when heated. Next time you sit by a campfire or use a wooden pencil, you can think about the amazing science behind this everyday material!
For more interesting articles, please visit www.kidzherald.com





